Acoustic (dis)similarity, coordinated singing and simulating songs
Marcelo Araya-Salas, PhD
Grace Smith-Vidaurre
2026-02-25
Source:vignettes/c_warbleR_workflow_02.Rmd
c_warbleR_workflow_02.RmdBioacoustics in R with warbleR

Bioacoustics research encompasses a wide range of questions, study
systems and methods, including the software used for analyses. The
warbleR and Rraven packages leverage the
flexibility of the R environment to offer a broad and
accessible bioinformatics tool set. These packages fundamentally rely
upon two types of data to begin bioacoustic analyses in R:
Sound files: Recordings in wav or mp3 format, either from your own research or open-access databases like xeno-canto
Selection tables: Selection tables contain the temporal coordinates (start and end points) of selected acoustic signals within recordings
Package repositories
These packages are both available on CRAN: warbleR,
Rraven,
as well as on GitHub: warbleR, Rraven. The
GitHub repository will always contain the latest functions and updates.
You can also check out an article in Methods in Ecology and
Evolution documenting the warbleR package
[1].
We welcome all users to provide feedback, contribute updates or new functions and report bugs to warbleR’s GitHub repository.
Please note that warbleR and Rraven use
functions from the seewave,
monitoR,
tuneR
and dtw
packages internally. warbleR and Rraven have
been designed to make bioacoustics analyses more accessible to
R users, and such analyses would not be possible without
the tools provided by the packages above. These packages should be given
credit when using warbleR and Rraven by
including citations in publications as appropriate
(e.g. citation("seewave")).
Parallel processing in warbleR
Parallel processing, or using multiple cores on your machine, can
greatly speed up analyses. All iterative warbleR functions
now have parallel processing for Linux, Mac and Windows operating
systems. These functions also contain progress bars to visualize
progress during normal or parallel processing. See
[1] for more details about improved
running time using parallel processing.
Vignette introduction
In this vignette we work on a case study of microgeographic vocal variation in long-billed hermit hummingbirds, Phaethornis longirostris [2] (and a short sidenote using Tinamus major for an example of a tonal signal) by:
Detecting signal frequency range
Extracting spectral entropy and frequency contours as time series
-
Comparing methods for quantitative analysis of signal structure
- data set of 29 acoustic parameters
- spectrographic cross-correlation
- dynamic time warping on frequency contours
Visually inspecting frequency contours
Measuring acoustic parameters as a batch-process across signals
Calculating pairwise acoustic (dis)similarity between signals
Analysis of geographic variation in Phaethornis longirostris songs
We also include some examples at the end of the vignette of how to perform coordinated singing analysis and simulate songs.
This vignette can be run without an advanced understanding of
R, as long as you know how to run code in your console.
However, knowing more about basic R coding would be very
helpful to modify the code for your research questions.
For more details about function arguments, input or output, read the
documentation for the function in question
(e.g. ?cross_correlation).
Prepare for analyses
library(warbleR)
# set your working directory appropriately
# setwd("/path/to/working directory")
# run this if you have restarted RStudio between vignettes without saving your workspace
# assumes that you are in your /home/username directory
setwd(file.path(getwd(), "warbleR_example"))
# Check your location
getwd()warbleR vignettes will not always include all available
warbleR functions, as existing functions are updated and
new functions are added. To see all functions available in this
package:
# The package must be loaded in your working environment
ls("package:warbleR")Extract acoustic parameters as time series
Detect frequency range
Raven selection tables can return low and high frequencies
in your selections (e.g. if all.data or
freq.cols in run_raven or
imp.raven is TRUE), but the accuracy of these frequency
selections depends on how the signals themselves were selected. Below we
demonstrate how to visualize and detect the frequency range in your
selected signals using the functions freq_range_detec and
freq_range, which have options for setting bandpass filters
to exclude background noise or other non-target acoustic signals.
freq_range_detec creates a plot that will print to your
graphics device, and also outputs a data frame per recording with the
frequency range. This data frame can be used in subsequent analyses if
saved as an object. freq_range_detec works best with single
signals. If used for a whole recording, freq_range_detec
will pick up all sounds in the recording.
Finally, although we have been using Phaethornis
longirostris vocalizations throughout this vignette, these signals
are harmonically structured. The functions for detecting frequency
ranges, freq_range and freq_range_detec work
best on tonal signals, so for this example we will use Great Tinamou (
Tinamus major) songs.
Download a tinamou recording from xeno-canto, make selections and visualize/detect frequency ranges.
tin <- query_xc(qword = "Tinamus", download = FALSE)
# select a single recording
tin <- tin[tin$Recordist == "Marcelo Araya-Salas", ]
# download this recording
query_xc(X = tin, download = TRUE)
mp32wav()
# here we will use a data set with sound files that have been already annotated
# read the selections back into the global environment
Tin.sels <- read.csv("manualoc_output.csv")
str(Tin.sels)
# cut the original wave file by selections for freq_range_detec below
writeWave(seewave::cutw(read_sound_file("Tinamus-major-154191.wav"), from = Tin.sels$start[1], to = Tin.sels$end[1], f = 44100, plot = FALSE, output = "Wave"), filename = "Tinamus-major-154191-1.wav")
writeWave(seewave::cutw(read_sound_file("Tinamus-major-154191.wav"), from = Tin.sels$start[2], to = Tin.sels$end[2], f = 44100, plot = FALSE, output = "Wave"), filename = "Tinamus-major-154191-2.wav")
# note that changing the threshold argument in combination with the bandpass argument can improve the detection
freq_range_detec(read_sound_file("Tinamus-major-154191-1.wav"), flim = c(0, 2.5), bp = c(0, 3), threshold = 15, plot = TRUE)
# here, giving a strict bandpass with very low threshold improves freq_range detection
# since the curving end of the tinamou signal is lower amplitude than the rest of the signal
c(read_sound_file("Tinamus-major-154191-1.wav"), flim = c(0, 2.5), bp = c(0, 3), threshold = 1, plot = TRUE)The function freq_range allows you to simultaneously
return the frequency ranges for all signals in a selection table,
including the graphical output as freq_range_detec. Check
out the resulting image file in your graphics device. In addition to
image files, this function returns the original selection table, as a
data frame with the newly calculated low and high frequency
measurements.
# use arguments from freq_range_detec above
fr <- freq_range(Tin.sels, threshold = 1, res = 100, flim = c(0, 2.5), bp = c(0.5, 2.5))
str(fr)Extract spectral entropy as a time series
Spectral entropy can be calculated as time series in selected signals
and plotted onto image files. Previously, spectral entropy was only
available as a sole measurement across a selection, as measured by
spectro_analysis. Check out the resulting image files in
your working directory.
Phae.hisnrt <- read.csv("Phae_hisnrt.csv", header = TRUE)
str(Phae.hisnrt)
se <- freq_ts(Phae.hisnrt, wl = 300, length.out = 10, threshold = 10, img = TRUE, img.suffix = "entropy_ts", type = "b", ovlp = 90, sp.en.range = c(-25, 10), flim = c(2, 10), picsize = 0.75, title = FALSE, type = "entropy")
str(se)Visualizing frequency contours with
track_freq_contour
The function track_freq_contour allows you to create
spectrograms and visualize the accuracy of dominant frequency and
fundamental frequency measurements.
Use track_freq_contour on all the recordings for which
you want to extract frequency contours as a time series, or later,
calculate other frequency measurements. Scroll through all the
spectrograms to get a feeling for how well the frequency measurements
will be performed across your recordings.
Running track_freq_contour can allow you to decide which
frequency parameters to use in subsequent analyses, namely
spectro_analysis and dynamic time warping methods. Also, ff
the frequency measurements look acceptable with the bandpass setting
used in track_freq_contour, use that same bandpass while
running spectro_analysis.
# Note that the dominant frequency measurements are almost always more accurate
track_freq_contour(Phae.hisnrt, wl = 300, flim = c(2, 10), bp = c(1, 12), it = "jpeg")
# We can change the lower end of bandpass to make the frequency measurements more precise
track_freq_contour(Phae.hisnrt, wl = 300, flim = c(2, 10), bp = c(2, 12), col = c("purple", "orange"), pch = c(17, 3), res = 100, it = "jpeg", picsize = 0.8)
Note that the fundamental frequency measurements are not always very accurate, so we will remove fundamental frequency measurements later on.
Extract fundamental or dominant frequency contours as a time series
These functions return a data frame that contains estimated frequency
contours as a time series across each signal in the input data frame.
You can also specify if you want to create image files with the
estimated frequency contours plotted over spectrograms. You can change
argument settings to better visualize the signals or change the
estimation of the frequency contour. For instance, the argument
threshold (as in auto_detec) controls the
amplitude threshold for estimating frequency values at each time point.
Note that the fundamental frequency contour estimation can often have
errors, and tends to perform best with more tonal signals. The frequency
contours are those that can be visualized using
track_freq_contour.
Manually tailor frequency contours with tailor_sels
The functions above to track/extract dominant and fundamental
frequency contours perform best with more tonal signals. The frequency
measurements for signals with harmonic structure tend to jump around,
and might not always match your own visual tracking of frequency
contours. If this is the case, you can use the function
tailor_sels to fix frequency contours where individual
frequency measurements are clearly far off from the frequency contour
detected by the human eye.
Note that manually fixing frequency contours might not make sense,
depending on your question and/or the contour in question. For instance,
a dominant frequency contour for a harmonic signal that jumps around and
does not form a smooth contour may in fact be the truth, rather than
mis-estimation of the contour. On the other hand, fundamental
frequencies can be more easily traced by the human eye across a signal,
so using tailor_sels to fix a frequency contour that jumps
around the signal makes more sense.
When the new graphics window for tailor_sels appears, it
will show spectrograms with frequency contours plotted as points over
each spectrogram. To fix the frequency contour, click near the
malaligned points to place them over the frequency contour that you
detect by eye. tailor_sels makes a new .csv
file in your working directory that merges your original data frame
(below, Phae.hisnrt) with the modified frequency time
series (below, ff_df with any modified frequency values).
To check that your manual tracing improved frequency contours, you can
use track_freq_contour to make spectrograms with your new
frequency contours plotted as custom contours.
# Use the original data frame of songs for the main tailor_sels dataset
# the data frame with the fundamental frequency contours is provided for manual tracing
tailor_sels(Phae.hisnrt,
wl = 300, flim = c(2, 10), wn = "hanning", mar = 0.1,
osci = TRUE, title = c("sound.files", "selec"), auto.contour = TRUE, ts.df = ff_df, col = "red", alpha = 0.6
)
# rename your tailor_sels output csv as desired, then read it back into R
mff <- read.csv("seltailor_output_mff.csv")
str(mff)
track_freq_contour(Phae.hisnrt, wl = 300, flim = c(2, 10), bp = c(1, 12), it = "jpeg", custom.contour = mff)Count inflections across frequency contours
This function calculates the modulation index for any frequency
contour or time series. The function spectro_analysis (see
below) also calculates a modulation index for signals, but as a single
value across the length of the signal.
df_inf <- inflections(X = df_df, pb = TRUE)
str(df_inf)##Quantitative measurements of acoustic (dis)similarity
Compare methods for quantitative analysis of signal structure
Bioacoustic research relies on quantifying the structure of acoustic signals and comparing that structure across behavioral/ecological contexts, groups or species. However, measuring signal structure in a way that fully accounts for the variation in the signals could be a tricky task. Some of the differences that are apparent by visual inspection of spectrograms might not be picked up by some analyses. Hence, choosing the most appropriate analytical approach is a critical step.
The warbleR function compare_methods
attempts to facilitate method selection. This function produces graphs
(as image files in the working directory) with spectrograms from 4
signals that allow visual inspection of the performance of acoustic
analysis methods at comparing those signals. The signals are randomly
picked up from the provided data frame (X argument), and
the function compares 2 warbleRmethods at a time. The
methods available are: * cross-correlation by warbleR function
cross_correlation * dynamic time warping on dominant or
fundamental frequency contours with freq_DTW * spectral
parameters with spectro_analysis
In the vignette “Visual inspection and signal classification”, we
tailored selections of Phaethornis longirostris songs that were
originally downloaded from xeno-canto, detected by
auto_detecand filtered by signal-to-noise ratio (SNR). Here
we will work on a data set of Phaethornis longirostris songs
that were originally downloaded from xeno-canto, using the data
frame Phae.hisnrt.
Phae.hisnrt <- read.csv("Phae_hisnrt.csv", header = TRUE)
compare_methods(
X = Phae.hisnrt, flim = c(0, 10), bp = c(0, 10),
wl = 300, n = 10, methods = c("XCORR", "dfDTW")
)compare_methods will produce 10 image files in the
working directory (since we specified n = 10) that look
like this:
In this graphic, the acoustic pairwise distance between signals is
shown next to the arrows linking them. The font color of a distance
value corresponds to the font color of the method that generated it, as
shown in the scatterplots (in this case black font represents XCORR
distances). Distances are standardized, with 0 being the distance of a
signal to itself and 1 the farthest pairwise distance in the pool of
signals. Principal Component Analysis (princomp function)
is applied to calculate distances when using spectral parameters (SP).
In this case, the first 2 PC’s are used. Classical Multidimensional
Scaling (also known as Principal Coordinates Analysis,
cmdscale function) is used for all other methods. The image
file name contains the methods being compared and the row number of the
selections. This function internally uses a modified version of the
spectro function from the seewave package to
create spectrograms. Note that the spectrograms are all plotted with the
same frequency and time scales.
Also note that the graphs contain 2 scatterplots (1 per method) of
the acoustic space of all signals in the input data frame
X. The position of the 4 signals in the spectrograms is
highlighted in the acoustic space scatterplot. These graphics allow you
to directly assess if the distances between signals in the acoustic
space accurately represent the spectrographic similarity (e.g. how
similar their acoustic structure looks in the spectrograms).
You can run compare_methods for any combination of the
quantitative methods for assessing acoustic (dis)similarity mentioned
above. Importantly, to include the SP method (spectral parameters
measured by the function spectro_analysis), you need a
large enough dataset, as the PCA that summarizes the spectral parameters
needs more units (rows) that variables (columns).
Measure acoustic parameters with spectro_analysis
We can now perform acoustic measurements with the function
spectro_analysis. This function relies on the temporal
coordinates in selection tables to measure 29 parameters across
selections. spectro_analysis is a batch process that is
faster than calculating measurements manually, e.g. one selection and
recording at a time. spectro_analysis uses and customizes
several functions available in the seewave package.
Use the bandpass filter to your advantage here, to filter out low or high background noise before performing measurements. Also note that changing the amplitude threshold will change the amplitude at which noises (including non-target signals) are detected for measurements.
params <- spectro_analysis(Phae.hisnrt, bp = c(2, 10), threshold = 15)
write.csv(params, "acoustic_parameters.csv", row.names = FALSE)Remove parameters derived from fundamental frequency (based on
track_freq_contour results).
Calculate acoustic parameters by song type
In addition to calculating acoustic parameters per individual signals
using spectro_analysis, you can also calculate these
acoustic parameters by song type (average, minimum and maximum values
per song type group).
data(list = c("Phae.long1", "Phae.long2", "Phae.long3", "Phae.long4", "lbh_selec_table"))
writeWave(Phae.long1, "Phae.long1.wav")
writeWave(Phae.long2, "Phae.long2.wav")
writeWave(Phae.long3, "Phae.long3.wav")
writeWave(Phae.long4, "Phae.long4.wav")
# Add a 'song' column
lbh_selec_table$song <- rep(1:4, each = 3)[1:11]
# Measure acoustic parameters
sp <- spectro_analysis(lbh_selec_table, bp = c(1, 11), 300, fast = TRUE)
# Add song data
sp <- merge(sp, lbh_selec_table, by = c("sound.files", "selec"))
# Caculate song-level parameters for all numeric parameters
sng <- song_analysis(X = sp, song_colm = "song", parallel = 1, pb = TRUE)
str(sng)Dynamic time warping of frequency contours
The dynamic time warping methods in warbleR all rely on
functions from the dtw package, and are available for both
dominant and fundamental frequencies. df_DTW and
ff_DTW calculate the dominant and fundamental frequency
contours, respectively, of each signal and compares using dynamic time
warping. You can interpolate measurements across the frequency time
series using the length.out argument.
These functions return a matrix of pairwise acoustic dissimilarity
(e.g. acoustic “distance”) measurements that can be used in analyses of
acoustic similarity, as well as image files with the frequency contours
plotted over the spectrograms. If you require only the time series
without the dynamic time warping analysis for either the dominant or
fundamental frequency, check out the functions freq_tsand
freq_ts.
Note that as the freq_range and
freq_range_detec functions, the dynamic time warping
functions tend to work best on more tonal signals. Check out the
resulting image files in your working directory.
Spectrographic cross-correlation
Cross-correlation calculates the pairwise similarity of multiple
signals by spectrogram cross-correlation. cross_correlation
calculates a correlation of the amplitude values at each step by sliding
one spectrogram over the other. This function is a modified version of
the corMatch and makeTemplate functions from
the package monitoR. cross_correlation runs
over multiple signals and returns a list of output, including:
- a correlation statistic for each “sliding” step
- the maximum (peak) correlation for each pairwise comparison
cross_correlation requires margins half the duration of
the signal on either side of the signal (e.g. before and after the start
and end coordinates). Signals that are very close to either the start or
end of a recording may throw errors. Additionally, when sound files have
been modified by bandpass filters, some pairwise cross-correlations may
not work and the correlation matrix may contain NA values.
cross_correlation now contains an argument to remove
columns of the matrix that contain NA values, which will
reduce the data in the matrix but also allow the matrix to be used in
subsequent analyses.
Here our output is a matrix of peak correlation per pairwise comparison, with the recording-selection names as the matrix dimension names.
xc <- cross_correlation(Phae.hisnrt, wl = 300, na.rm = FALSE)
str(xc)Analysis of geographic variation using spectro_analysis
measurements
We can evaluate whether or not the observed variation in song structure is reflected by the meters we just measured. For this we will conduct a Principal Component Analysis on scaled (z-transformed) meters and look at the grouping of songs (data points) in the scatter plot.
# Run the PCA with only numeric variables of params
pca <- prcomp(x = params[, sapply(params, is.numeric)], scale. = TRUE)
# Check loadings
summary(pca)Importance of components:
PC1 PC2 PC3 PC4 PC5 PC6 PC7 PC8 PC9 PC10 PC11 PC12 PC13 PC14 PC15 PC16
Standard deviation 3.799 2.6465 1.7684 1.01130 0.93681 0.88418 0.8449 0.72613 0.63069 0.52078 0.44692 0.43914 0.41945 0.3552 0.23923 0.18924
Proportion of Variance 0.481 0.2335 0.1042 0.03409 0.02925 0.02606 0.0238 0.01758 0.01326 0.00904 0.00666 0.00643 0.00586 0.0042 0.00191 0.00119
Cumulative Proportion 0.481 0.7145 0.8187 0.85280 0.88205 0.90811 0.9319 0.94948 0.96274 0.97178 0.97844 0.98487 0.99073 0.9949 0.99685 0.99804
PC17 PC18 PC19 PC20 PC21 PC22 PC23
Standard deviation 0.15921 0.15130 0.07228 0.05806 0.03699 0.02449 2.227e-15
Proportion of Variance 0.00084 0.00076 0.00017 0.00011 0.00005 0.00002 0.000e+00
Cumulative Proportion 0.99888 0.99965 0.99982 0.99993 0.99998 1.00000 1.000e+00
# Extract PCA scores
pcascor <- as.data.frame(pca[[5]])
# Plot the 2 first PCs
plot(pcascor[, 1], pcascor[, 2],
col = as.numeric(as.factor(params$sound.files)), pch = 20,
cex = 1, xlab = "PC1", ylab = "PC2"
)
# Add recordings/individuals labels
x <- tapply(pcascor[, 1], params$sound.files, mean)
y <- tapply(pcascor[, 2], params$sound.files, mean)
labs <- gsub(".wav", "", unique(sapply(as.character(params$sound.files), function(x) {
strsplit(x, split = "-", fixed = TRUE)[[1]][3]
}, USE.NAMES = FALSE)))
text(x, y, labs, cex = 0.75)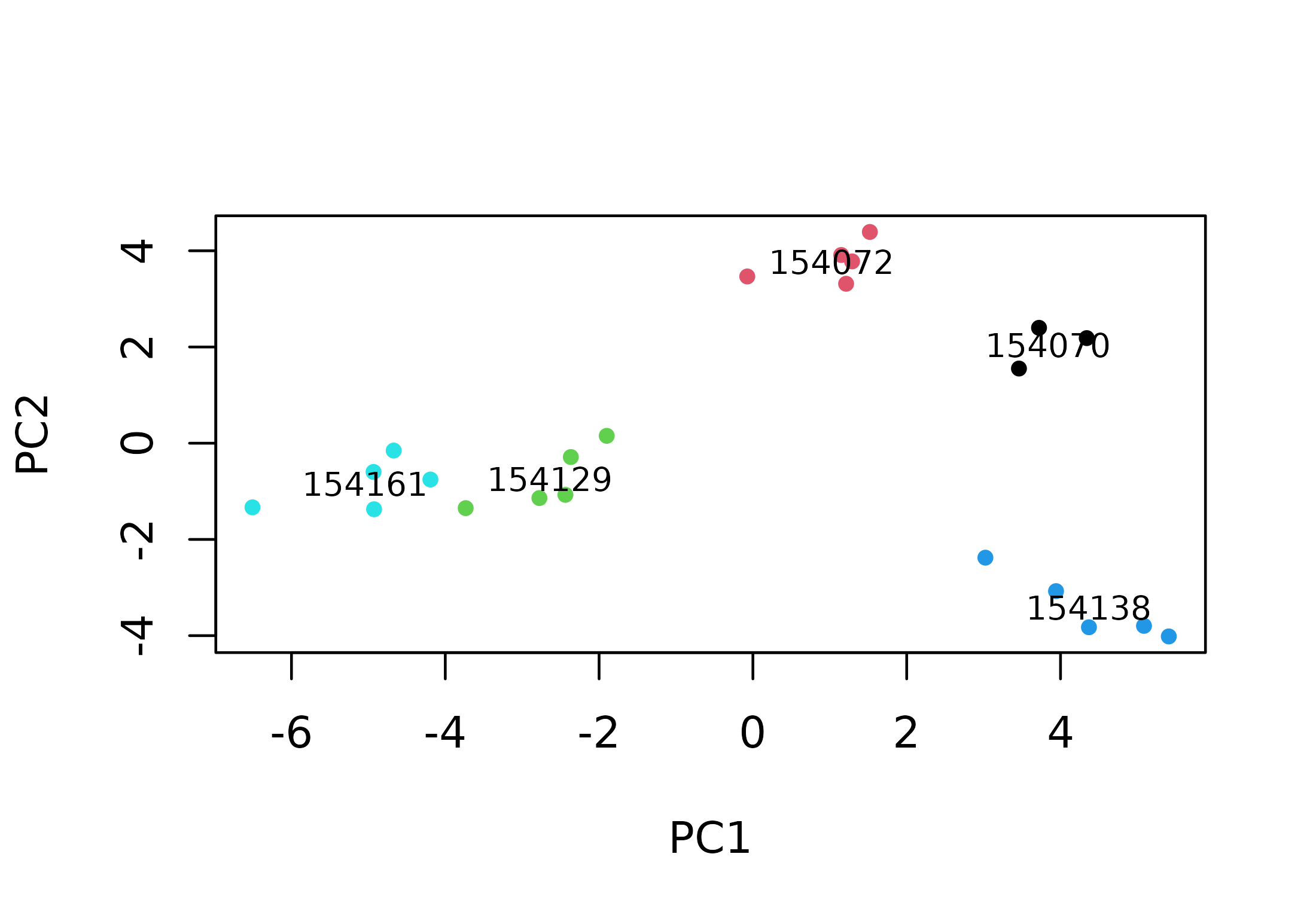
Songs are grouped by sound file. As each sound file represents a single individual, this suggests that songs have individual signatures. Let’s look at the song type level. First, we need to classify the songs by song type. We can check the spectrograms we previously created to do this.
Songs from sound files 154070 and 154072 seem to belong to the same song type. Sound files 154129 and 154161 represent a different song type. Finally, the songs from each of the other 2 sound files have a unique structure, so each one represents a different song type. We can add this information to the plot by using symbols to represent song types.
# Create a song type variable
# First, extract recording ID
songtype <- gsub(".wav", "", sapply(as.character(params$sound.files), function(x) {
strsplit(x, split = "-", fixed = TRUE)[[1]][3]
}, USE.NAMES = FALSE))
# Now change IDs for letters representing song types
songtype <- gsub("154070|154072", "A", songtype)
songtype <- gsub("154129|154161", "B", songtype)
songtype <- gsub("154138", "C", songtype)
# Add song type as a variable representing symbol type
plot(pcascor[, 1], pcascor[, 2],
col = as.numeric(as.factor(params$sound.files)),
pch = as.numeric(as.factor(songtype)),
cex = 1, xlab = "PC1", ylab = "PC2"
)
# Add song type labels
x <- tapply(pcascor[, 1], songtype, mean)
y <- tapply(pcascor[, 2], songtype, mean)
text(x, y, unique(songtype), cex = 1)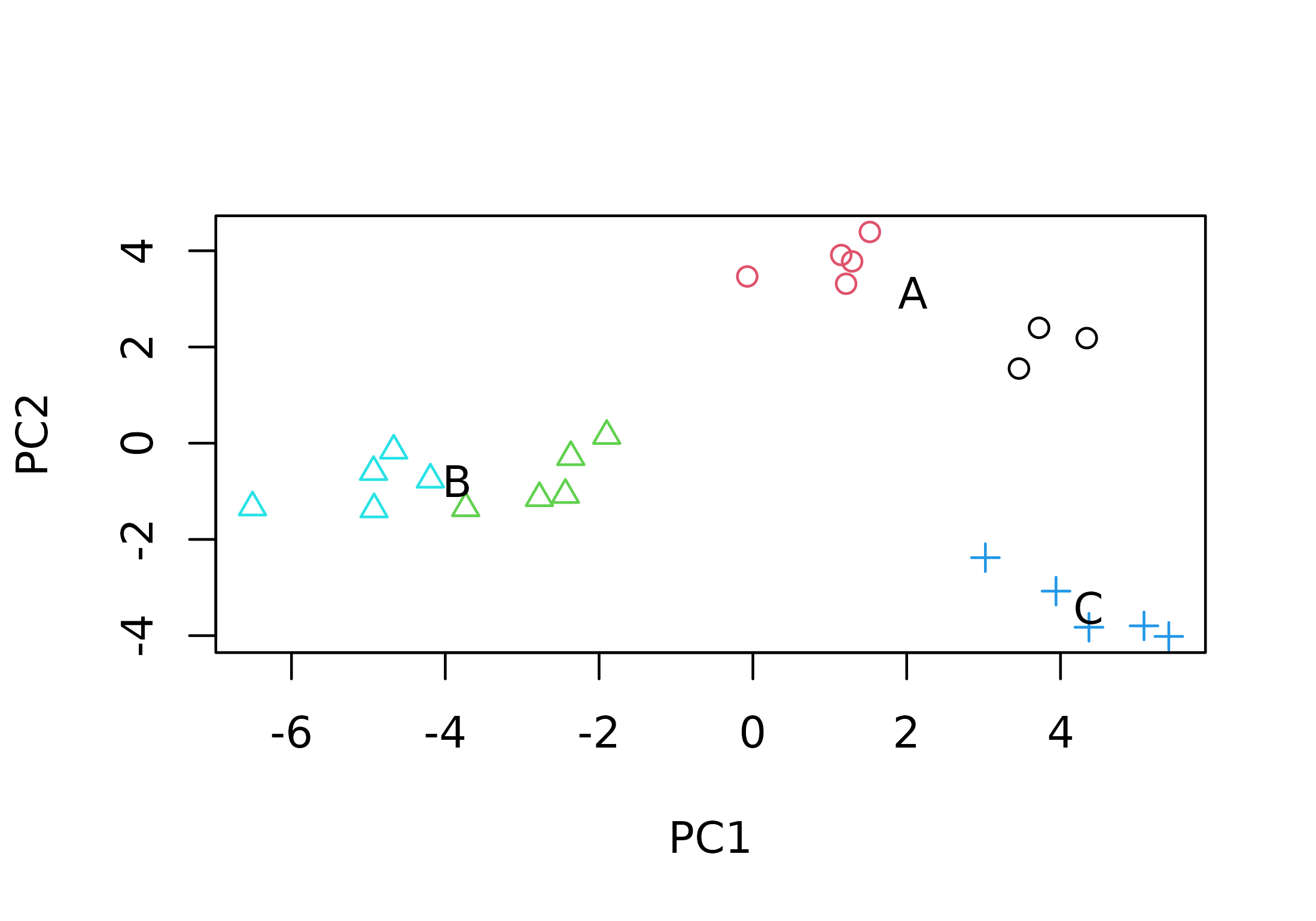
Songs of the same song type are more similar (they cluster together).
This PCA confirms that the visually obvious differences in the song
structures are well described by the meters measured in warbleR.
Likewise, it also confirms that we can detect biologically relevant
differences from sound files that have undergone mp3
compression and conversion back to wav format (see also
[2]). Importantly, if this
analysis were for publication we would have to run other filtering steps
on the acoustic parameters generated by spectro_analysis,
including removing any collinear variables.
Coordinated singing analysis
warbleR contains functions to visualize and test whether
or not singing bouts between individuals can be considered coordinated
singing. The function plot_coordination plots signals as
polygons to better visualize overlap between these signals.
The function test_coordination uses a Monte Carlo
randomization test to determine whether or not the probability of
finding overlapping songs in a singing event is less than or more than
what you would expect by chance. test_coordination can be
run simultaneously for many singing events, if all are contained in a
single data frame (see test_coordination documentation for
more information).
The sim_coor_sing dataset contains three types of
singing bouts:
# save plots in a list
g <- plot_coordination(sim_coor_sing, it = "jpeg", img = FALSE, res = 300)
# print list of plots to graphics device
gWe can test for overlapping or alternating coordinated singing using
the less.than.chance argument in
test_coordination. Here we will test for alternating
coordinated singing, since less.than.chance is set to
TRUE.
cs <- test_coordination(sim_coor_sing, iterations = 1000, less.than.chance = TRUE, cutoff = 10)
str(cs)Simulating songs
You can simulate songs using warbleR. Depending on your
question, you can simulate songs with different numbers of subunits,
subunit durations, numbers of harmonics, amplitudes, gaps between
subunits, amplitude fading of subunits, among other options. Songs are
simulated under Brownian motion frequency drift.
# simulate a song with 3 tonal elements
ss <- simulate_songs(n = 3, harms = 1)
# plot the simulated song
# seewave::spectro(ss)
# simulate a song with 3 harmonic elements of differing amplitude
ss <- simulate_songs(n = 3, harms = 3)
# plot the simulated song
seewave::spectro(ss)
Citation
Please cite warbleR when you use the package:
Araya-Salas, M. and Smith-Vidaurre, G. (2017), warbleR: an R package to streamline analysis of animal acoustic signals. Methods Ecol Evol. 8, 184-191.
Reporting bugs
Please report any bugs here.